Safer Surgery Saves Lives
GS1 Identifcation and bar code standards deployed in the Irish Health
Service Executive’s (HSE) Central Decontamination Units (CDUs)
ABSTRACT
The Health Service Executive (HSE) is the government agency responsible for the provision of public healthcare services for everyone living in Ireland. The HSE now requires that all surgical instrument trays are identifed using GS1 Standards to enable stakeholders to track and trace them throughout the supply chain. The system currently being rolled out is designed to create a collaborative, interoperable, and nationwide traceability solution for Central Decontamination Units.

by Pauline Biggane
Introduction
There is well documented evidence highlighting the importance of effective decontamination processes to prevent the spread of infections. The Medical Devices Directive (93/42/EEC) specifes the minimum standards in relation to decontamination of Reusable Invasive Medical Devices. Hospital acquired infections are a concern for all hospitals and their patients as Surgical Site Infections (SSIs) can have an impact on both patient safety (e.g., development of a serious illness) and hospital costs (e.g., additional cost of treatment for the patient). The importance of a robust track and trace system that complies with regional, national and international best practices for the decontamination of surgical
instrument sets is recognised as an integral part of all Central Decontamination Units (CDUs). Under the current economic and budgetary pressures that are faced by most health services across the globe, including Ireland, there is often a need to share important hospital resources such as surgical instrument sets. There is also a signifcant market for manufacturers to loan instrument sets to hospitals for specifc procedures because it is not cost effective for a hospital to purchase them.
Proof of Concept
A Proof of Concept (POC) began in 2011. The objective was clear: could the use of GS1 Standards help realise the requirement of the HSE to increase Patient Safety by introducing an interoperable traceability system for the Irish National Health Service? St. James’s Hospital volunteered to demonstrate the feasibility of the project. St. James’s is a leading, major acute and academic teaching hospital. The hospital caters for over 14,000 surgically invasive procedures every year. With 30,000 surgical trays, including over 300 shared loan sets being reprocessed by St. James’s CDU every year, the POC was needed to reflect the complex nature of implementing a national traceability solution. The POC showed that by implementing GS1 Standards the CDU moved away from manual processes, and at the same time, effectively managed a 17% increase in workload with less staff members. Following the successful pilot at St. James’s Hospital, the HSE made the decision to roll out the system in seven additional HSE and HSE-funded acute hospitals throughout Ireland in 2012:
Sharing reusable surgical instrument sets has obvious benefts, but can challenge proprietary track and trace systems. Having multiple proprietary systems in each hospital can result in a break in the traceability chain when an item leaves a hospital, as there is no guarantee that the identifcation of that item is unique. Recognising this challenge, the Health Service Executive (HSE) in Ireland implemented a solution using GS1 Standards.
Ireland - Safer Surgery Saves Lives
- Beaumont Hospital
- Kerry General Hospital
- Mullingar General Hospital
- Portlaoise General Hospital
- Adelaide and Meath Hospital, incorporating the National Children’s Hospital
- Tullamore General Hospital
- Waterford Regional Hospital
A simple solution to a complex problem
The supply and demand chains for surgical instrument sets are complex. There are two distribution channels needed to get the product to the end user: a surgeon or another medical professional. Firstly there is the macro supply chain; when an instrument set is loaned between hospitals or when it is loaned or consigned to a hospital from a commercial loan set provider. Secondly there is the micro distribution channel; the decontamination process that occurs within the four walls of the CDU. Importantly, continuity of traceability between these two supply chains is a key factor in ensuring patient safety.
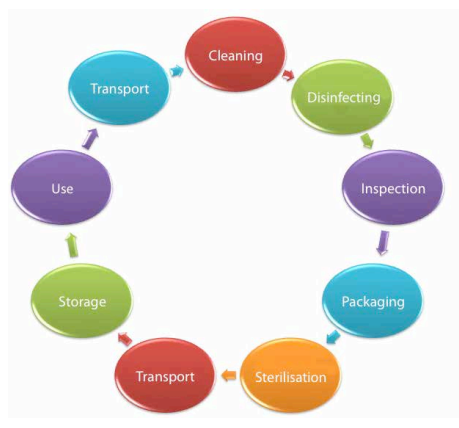
Using GS1 Standards of Identifcation
GS1 Standards and a new electronic traceability system was introduced in eight hospitals initially. A GS1 Global Individual Asset Identifer (GIAI) was assigned to each instrument tray owned by the hospital. As the tray moves through the decontamination process, its unique identity is enhanced. Batch numbers identify the relevant autoclave cycle, expiration dates help ensure the surgical instruments are decontaminated when necessary and Global Location Numbers (GLN) are used to identify the location of the CDU that has decontaminated the surgical instrument set and its contents. Each step of the process is time and date stamped when the GS1 bar code is scanned. Technicians working in the CDU scan their ID badges to complete the “who, what, where and when” of the traceability process.
The Traceability Process
The track and trace software system application is provided by FingerPrint Medical Ltd. The system helps users monitor all control points in the decontamination supply chain, from using an autoclave to decontaminate the instruments in the CDU to linking a particular instrument set to a patient in theatre, by scanning the GS1 bar code at each of the control points. In addition to providing the checks and balances that help create the assurances needed for patient safety, the software helps its users to drive efciencies in their departments.
An Independent Monitoring System (IMS), which monitors the Autoclaves (Temperature & Water Pressure) and Washer-Disinfectors (Temperature, Dose Levels & Conductivity), is also required by the HSE. The IMS functionality was provided by Irish Power and Process and interfaced seamlessly with the track and trace application, adding an additional layer of quality assurance. Signifcantly, if any cycle fails to reach the required decontamination parameters this is immediately communicated to the track and trace system. When the bar code is scanned at the relevant control point, the user is notifed if there was a failure in the autoclave process, thus requiring the instruments to begin the decontamination process again.
Sláinte Healthcare provided the shadow Patient Master Index (PMI) for this project. The shadow PMI enables theatre staff to ensure the correct patient is in the operating theatre and to record the exact instruments used for the patient’s procedure. This is done by reading feeds of demographic data from the hospital administration system and making the information available to the track and trace software. The instrument tray is scanned against the patient’s procedure and the data is recorded accordingly. Finally, hospitals and commercial loan set providers can share the transactional data needed for their business processes by using the cloud based Medical Standards 1 (MS1) software, which is also integrated with the track and trace software. Up-to-date data on the contents of the instrument trays is accessed when the bar code is scanned on the instrument set. The system enables hospitals to download up-to-date instruments tray contents, decontamination certifcates and instructional photographs to streamline processes.

Ireland - Safer Surgery Saves Lives

The traceability chain is also maintained when an instrument tray is loaned between hospitals using the track and trace system. When a hospital borrows an instrument set, they simply scan the GS1 bar code which acts as the key to access the relevant information. The details associated with each particular instrument set can then be downloaded to the local systems, maintaining traceability data and ensuring accurate data on the contents of the set. Prior to the adoption of GS1 Standards this was a paper-based, error prone, and labour intensive process.
The HSE has published three documents that explicitly recommend the use of GS1 Standards to help them realise their exacting standards. These documents describe a set of standards which defne the structures and processes needed to identify, assess and manage specifed risks in relation to the decontamination process.
Nothing succeeds like success
The HSE has steered this project with great success. The natural next step for the project was to extend GS1 coding to ridged and flexible endoscopes. The risk involved, both to patient and provider, in the use and maintenance of these instruments is well documented and GS1 Identifcation Standards are now being rolled out in Endoscope Reprocessing Units (ERUs). The use of GS1 Standards is viewed as a means by which assurances about the integrity of the HSE’s standards can be automatically captured and shared amongst all stakeholders, thereby helping to streamline ERUs in the same way that the HSE has accomplished with instrument tray management in CDUs.
Benefts and outcomes
The objectives of the project (to reduce manual labour, increase efciency and create assurances that an effective decontamination process has occurred) have all been achieved through the track and trace solution. For both patients and internal customers of the CDU, the implementation of the traceability solution and the adoption of GS1 Standards have exceeded expectations and many unanticipated benefts have also been achieved. Indeed, one of the biggest benefts identifed is the ability to loan instruments seamlessly between the eight hospitals currently participating in the initiative. Ultimately the full beneft will be fulflled when manufacturers apply the GS1 Identifer at the point of manufacture. In this regard, DePuy Synthes is the frst manufacturer to mark their loaned tray sets at source, to guarantee traceability from manufacturing site right through to the patient record.
Ireland - Safer Surgery Saves Lives
Prior to the implementation of the traceability system, using loan sets for a specifc procedure could involve the reprocessing of between 10 to 15 individual sets of instruments before and after the procedure. The contents of the loan set also had to be manually entered into the track and trace software, sometimes taking staff an additional 3 to 4 hours to complete the task. DePuy Synthes’ use of GS1 Standards has delivered a signifcant reduction in the manual work that hospitals encounter when receiving and processing instrument trays.
The success of the project, the benefts delivered and the willingness of DePuy Synthes to foster a collaborative traceability solution with the HSE has not gone unnoticed. The HSE is now in communication with the majority of commercial loan set providers in Ireland and hopes to work closely with them. Encouragingly, the commercial loan set providers engaged in the project recognise both the business and the patient safety benefts that using GS1 Standards can enable.
The reality is that managing inventory is a difcult task for hospitals, instrument manufacturers and distributers alike. It is a cumbersome, resource intensive process that is complicated by the fact that an instrument tray’s visibility is critical to patient safety and the efciency of a CDU. Independent research conducted by Trinity College Dublin identifed some compelling benefts of implementing a collaborative interoperable solution:
Patient safety benefts:
- Robust traceability of instrument sets with audit trails for quality assurance are electronically accessible
- Instrument sets can be located quickly in emergency situations
- Warnings are provided if a step is skipped in the decontamination process
- Links between patients, instrument sets and the decontamination process are established
Efciency benefts:
- Ability to analyse staff productivity to improve processes
- Ease of reporting both during and post event
- Automated validation and streamlined processes
- Inventory visibility available in real time
- Automatic generation of set lists when the GS1 code is scanned, reducing administrative work
- Improved communication between CDU and theatre staff, ensuring sets are ready where and when needed
The independent research also found that the potential savings in economic terms are twofold. Firstly, if 30%1 of Hospital Acquired Infections (HAI) is caused by Surgical Site Infections (SSI), and if an estimated 10%1 of these are attributable to contaminated surgical instruments, the potential cost savings from implementing the traceability system means the system would pay for itself in the frst year. It was also established that by reducing SSIs hospitals may have more beds available for patients that need them. Perhaps most importantly, the impact of stress, longer hospital stays and adverse events on the health of patients can also be reduced.
The Future
The success of implementing GS1 Standards for tracing instrument trays between and within Irish hospitals has highlighted that a combination of effective processes, the application of GS1 Standards and use of scanning technologies have measurable benefts for all Healthcare stakeholders. The instrument track and trace solution incorporating GS1 Standards is currently being rolled out to an additional 31 Hospitals. The scope of the current phase of the project now includes endoscope reprocessing sites, which will bring further benefts by virtue of scale and patient safety. Further phases will involve single instrument marking, helping to ensure a level of traceability and reporting that would not have been previously possible with a manual or proprietary system of identifcation.
About the Author
Pauline Biggane is a project analyst with the ICT Directorate of the HSE. Currently one of the projects Pauline is managing is the National Reusable Invasive Medical Devices (RIMD) / Endoscope Track and Trace Project. Working with clinicians and other healthcare professionals, Pauline has procured and implemented clinical IT solutions across acute hospitals in the HSE South East region for the beneft of patient safety and the improvement of service delivery.
Pauline is trained as a Nurse and specialised in Midwifery. She holds a degree in Healthcare Management with the Institute of Public Administration. Through both her academic and practical experience, Pauline has developed expertise in change management, Project planning, implementation and business process modelling.
Co-author
Alan Gormley is a Technical Expert in the Automatic Identifcation and Data Capture (AIDC) aspects of the GS1 System® and also responsible for the development and delivery of training, at GS1 Ireland.
Collaborating with solution providers nationally and globally, Alan works in numerous industries such as healthcare, aerospace and retail to help implement GS1 Standards for traceability, patient safety and efciency solutions. Alan holds diplomas in both process and polymer engineering, in addition to a Bachelor’s Degree in Economics and Law from University College, Galway (NUIG) and a Master’s Degree from Trinity College, Dublin.

