Safer Surgery Saves Lives
GS1 Identifcation and bar code standards deployed in the Irish Health
Service Executive’s (HSE) Central Decontamination Units (CDUs)
ABSTRACT
The Health Service Executive (HSE) is the government agency responsible for the provision of public healthcare services for everyone living in Ireland. The HSE now requires that all surgical instrument trays are identifed using GS1 Standards to enable stakeholders to track and trace them throughout the supply chain. The system currently being rolled out is designed to create a collaborative, interoperable, and nationwide traceability solution for Central Decontamination Units.

by Pauline Biggane
Introduction
There is well documented evidence highlighting the importance of effective decontamination processes to prevent the spread of infections. The Medical Devices Directive (93/42/EEC) specifes the minimum standards in relation to decontamination of Reusable Invasive Medical Devices. Hospital acquired infections are a concern for all hospitals and their patients as Surgical Site Infections (SSIs) can have an impact on both patient safety (e.g., development of a serious illness) and hospital costs (e.g., additional cost of treatment for the patient). The importance of a robust track and trace system that complies with regional, national and international best practices for the decontamination of surgical
instrument sets is recognised as an integral part of all Central Decontamination Units (CDUs). Under the current economic and budgetary pressures that are faced by most health services across the globe, including Ireland, there is often a need to share important hospital resources such as surgical instrument sets. There is also a signifcant market for manufacturers to loan instrument sets to hospitals for specifc procedures because it is not cost effective for a hospital to purchase them.
Proof of Concept
A Proof of Concept (POC) began in 2011. The objective was clear: could the use of GS1 Standards help realise the requirement of the HSE to increase Patient Safety by introducing an interoperable traceability system for the Irish National Health Service? St. James’s Hospital volunteered to demonstrate the feasibility of the project. St. James’s is a leading, major acute and academic teaching hospital. The hospital caters for over 14,000 surgically invasive procedures every year. With 30,000 surgical trays, including over 300 shared loan sets being reprocessed by St. James’s CDU every year, the POC was needed to reflect the complex nature of implementing a national traceability solution. The POC showed that by implementing GS1 Standards the CDU moved away from manual processes, and at the same time, effectively managed a 17% increase in workload with less staff members. Following the successful pilot at St. James’s Hospital, the HSE made the decision to roll out the system in seven additional HSE and HSE-funded acute hospitals throughout Ireland in 2012:
Sharing reusable surgical instrument sets has obvious benefts, but can challenge proprietary track and trace systems. Having multiple proprietary systems in each hospital can result in a break in the traceability chain when an item leaves a hospital, as there is no guarantee that the identifcation of that item is unique. Recognising this challenge, the Health Service Executive (HSE) in Ireland implemented a solution using GS1 Standards.
Ireland - Safer Surgery Saves Lives
- Beaumont Hospital
- Kerry General Hospital
- Mullingar General Hospital
- Portlaoise General Hospital
- Adelaide and Meath Hospital, incorporating the National Children’s Hospital
- Tullamore General Hospital
- Waterford Regional Hospital
A simple solution to a complex problem
The supply and demand chains for surgical instrument sets are complex. There are two distribution channels needed to get the product to the end user: a surgeon or another medical professional. Firstly there is the macro supply chain; when an instrument set is loaned between hospitals or when it is loaned or consigned to a hospital from a commercial loan set provider. Secondly there is the micro distribution channel; the decontamination process that occurs within the four walls of the CDU. Importantly, continuity of traceability between these two supply chains is a key factor in ensuring patient safety.
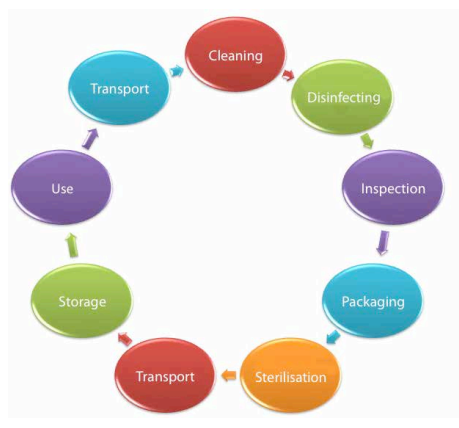
Using GS1 Standards of Identifcation
GS1 Standards and a new electronic traceability system was introduced in eight hospitals initially. A GS1 Global Individual Asset Identifer (GIAI) was assigned to each instrument tray owned by the hospital. As the tray moves through the decontamination process, its unique identity is enhanced. Batch numbers identify the relevant autoclave cycle, expiration dates help ensure the surgical instruments are decontaminated when necessary and Global Location Numbers (GLN) are used to identify the location of the CDU that has decontaminated the surgical instrument set and its contents. Each step of the process is time and date stamped when the GS1 bar code is scanned. Technicians working in the CDU scan their ID badges to complete the “who, what, where and when” of the traceability process.
The Traceability Process
The track and trace software system application is provided by FingerPrint Medical Ltd. The system helps users monitor all control points in the decontamination supply chain, from using an autoclave to decontaminate the instruments in the CDU to linking a particular instrument set to a patient in theatre, by scanning the GS1 bar code at each of the control points. In addition to providing the checks and balances that help create the assurances needed for patient safety, the software helps its users to drive efciencies in their departments.
An Independent Monitoring System (IMS), which monitors the Autoclaves (Temperature & Water Pressure) and Washer-Disinfectors (Temperature, Dose Levels & Conductivity), is also required by the HSE. The IMS functionality was provided by Irish Power and Process and interfaced seamlessly with the track and trace application, adding an additional layer of quality assurance. Signifcantly, if any cycle fails to reach the required decontamination parameters this is immediately communicated to the track and trace system. When the bar code is scanned at the relevant control point, the user is notifed if there was a failure in the autoclave process, thus requiring the instruments to begin the decontamination process again.
Sláinte Healthcare provided the shadow Patient Master Index (PMI) for this project. The shadow PMI enables theatre staff to ensure the correct patient is in the operating theatre and to record the exact instruments used for the patient’s procedure. This is done by reading feeds of demographic data from the hospital administration system and making the information available to the track and trace software. The instrument tray is scanned against the patient’s procedure and the data is recorded accordingly. Finally, hospitals and commercial loan set providers can share the transactional data needed for their business processes by using the cloud based Medical Standards 1 (MS1) software, which is also integrated with the track and trace software. Up-to-date data on the contents of the instrument trays is accessed when the bar code is scanned on the instrument set. The system enables hospitals to download up-to-date instruments tray contents, decontamination certifcates and instructional photographs to streamline processes.

Ireland - Safer Surgery Saves Lives

The traceability chain is also maintained when an instrument tray is loaned between hospitals using the track and trace system. When a hospital borrows an instrument set, they simply scan the GS1 bar code which acts as the key to access the relevant information. The details associated with each particular instrument set can then be downloaded to the local systems, maintaining traceability data and ensuring accurate data on the contents of the set. Prior to the adoption of GS1 Standards this was a paper-based, error prone, and labour intensive process.
The HSE has published three documents that explicitly recommend the use of GS1 Standards to help them realise their exacting standards. These documents describe a set of standards which defne the structures and processes needed to identify, assess and manage specifed risks in relation to the decontamination process.
Nothing succeeds like success
The HSE has steered this project with great success. The natural next step for the project was to extend GS1 coding to ridged and flexible endoscopes. The risk involved, both to patient and provider, in the use and maintenance of these instruments is well documented and GS1 Identifcation Standards are now being rolled out in Endoscope Reprocessing Units (ERUs). The use of GS1 Standards is viewed as a means by which assurances about the integrity of the HSE’s standards can be automatically captured and shared amongst all stakeholders, thereby helping to streamline ERUs in the same way that the HSE has accomplished with instrument tray management in CDUs.
Benefts and outcomes
The objectives of the project (to reduce manual labour, increase efciency and create assurances that an effective decontamination process has occurred) have all been achieved through the track and trace solution. For both patients and internal customers of the CDU, the implementation of the traceability solution and the adoption of GS1 Standards have exceeded expectations and many unanticipated benefts have also been achieved. Indeed, one of the biggest benefts identifed is the ability to loan instruments seamlessly between the eight hospitals currently participating in the initiative. Ultimately the full beneft will be fulflled when manufacturers apply the GS1 Identifer at the point of manufacture. In this regard, DePuy Synthes is the frst manufacturer to mark their loaned tray sets at source, to guarantee traceability from manufacturing site right through to the patient record.
Ireland - Safer Surgery Saves Lives
Prior to the implementation of the traceability system, using loan sets for a specifc procedure could involve the reprocessing of between 10 to 15 individual sets of instruments before and after the procedure. The contents of the loan set also had to be manually entered into the track and trace software, sometimes taking staff an additional 3 to 4 hours to complete the task. DePuy Synthes’ use of GS1 Standards has delivered a signifcant reduction in the manual work that hospitals encounter when receiving and processing instrument trays.
The success of the project, the benefts delivered and the willingness of DePuy Synthes to foster a collaborative traceability solution with the HSE has not gone unnoticed. The HSE is now in communication with the majority of commercial loan set providers in Ireland and hopes to work closely with them. Encouragingly, the commercial loan set providers engaged in the project recognise both the business and the patient safety benefts that using GS1 Standards can enable.
The reality is that managing inventory is a difcult task for hospitals, instrument manufacturers and distributers alike. It is a cumbersome, resource intensive process that is complicated by the fact that an instrument tray’s visibility is critical to patient safety and the efciency of a CDU. Independent research conducted by Trinity College Dublin identifed some compelling benefts of implementing a collaborative interoperable solution:
Patient safety benefts:
- Robust traceability of instrument sets with audit trails for quality assurance are electronically accessible
- Instrument sets can be located quickly in emergency situations
- Warnings are provided if a step is skipped in the decontamination process
- Links between patients, instrument sets and the decontamination process are established
Efciency benefts:
- Ability to analyse staff productivity to improve processes
- Ease of reporting both during and post event
- Automated validation and streamlined processes
- Inventory visibility available in real time
- Automatic generation of set lists when the GS1 code is scanned, reducing administrative work
- Improved communication between CDU and theatre staff, ensuring sets are ready where and when needed
The independent research also found that the potential savings in economic terms are twofold. Firstly, if 30%1 of Hospital Acquired Infections (HAI) is caused by Surgical Site Infections (SSI), and if an estimated 10%1 of these are attributable to contaminated surgical instruments, the potential cost savings from implementing the traceability system means the system would pay for itself in the frst year. It was also established that by reducing SSIs hospitals may have more beds available for patients that need them. Perhaps most importantly, the impact of stress, longer hospital stays and adverse events on the health of patients can also be reduced.
The Future
The success of implementing GS1 Standards for tracing instrument trays between and within Irish hospitals has highlighted that a combination of effective processes, the application of GS1 Standards and use of scanning technologies have measurable benefts for all Healthcare stakeholders. The instrument track and trace solution incorporating GS1 Standards is currently being rolled out to an additional 31 Hospitals. The scope of the current phase of the project now includes endoscope reprocessing sites, which will bring further benefts by virtue of scale and patient safety. Further phases will involve single instrument marking, helping to ensure a level of traceability and reporting that would not have been previously possible with a manual or proprietary system of identifcation.
About the Author
Pauline Biggane is a project analyst with the ICT Directorate of the HSE. Currently one of the projects Pauline is managing is the National Reusable Invasive Medical Devices (RIMD) / Endoscope Track and Trace Project. Working with clinicians and other healthcare professionals, Pauline has procured and implemented clinical IT solutions across acute hospitals in the HSE South East region for the beneft of patient safety and the improvement of service delivery.
Pauline is trained as a Nurse and specialised in Midwifery. She holds a degree in Healthcare Management with the Institute of Public Administration. Through both her academic and practical experience, Pauline has developed expertise in change management, Project planning, implementation and business process modelling.
Co-author
Alan Gormley is a Technical Expert in the Automatic Identifcation and Data Capture (AIDC) aspects of the GS1 System® and also responsible for the development and delivery of training, at GS1 Ireland.
Collaborating with solution providers nationally and globally, Alan works in numerous industries such as healthcare, aerospace and retail to help implement GS1 Standards for traceability, patient safety and efciency solutions. Alan holds diplomas in both process and polymer engineering, in addition to a Bachelor’s Degree in Economics and Law from University College, Galway (NUIG) and a Master’s Degree from Trinity College, Dublin.
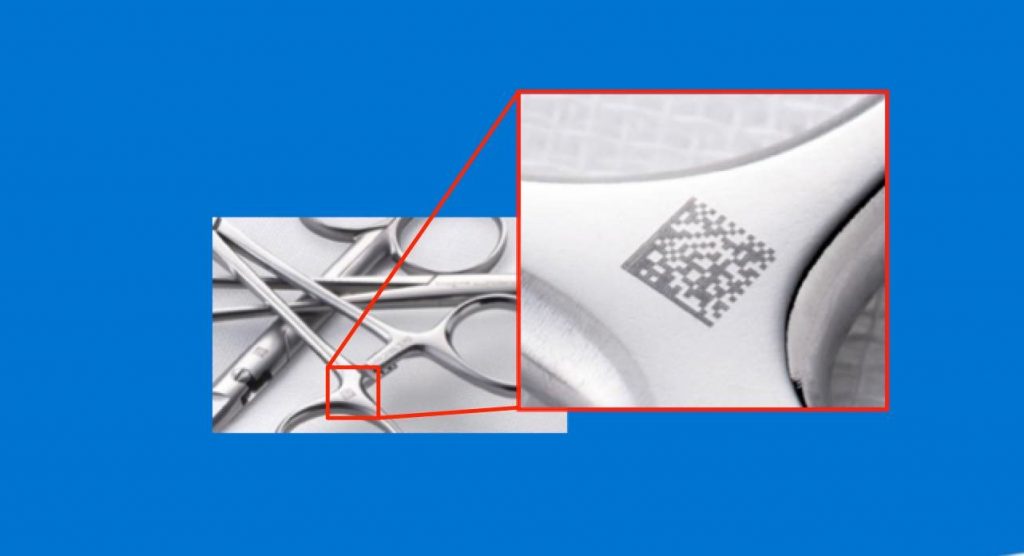
大家好,我的名字叫矢泽贤裕,今天我的演讲主题是关于:DPM刻印技术在手术器械追溯及质量控制上的运用。
那么再来介绍一下我自己。除了就职于罗兰公司之外,我个人也是日本医学工会的委员,参与工会的业务活动,同时,我也参与了创建刻印技术的标准制定和技术指导。“日本医疗器械行业协会DPM委员会委员 (JAMDI)”日本医疗仪器协会UDI标准化委员会委员(JSMI) 我同时也是日本GS-1卫生保健协会DPM委员会副主任,参与医疗金属器材操作指南的制定。下面我就来分享一下我这么多年来积累的行业经验,以及DPM刻印技术在手术器械追溯及质量控制上的运用。

让我们再来看一下经过DPM刻印后的医疗器械有什么用。

有各种类型的医疗器械被用于各家医院。

我想告诉大家的是,经DPM刻印后的医疗器械结合可追溯系统,将会大大提高工作率。

人无完人,有些失误是在不经意间发生的。实际上消毒失误会带来很多安全隐患。
1. 高昂的修理费用的发生。
2. 因发现消毒问题造成手术的中止,更有可能会给患者带来更大的伤害。

那么我们再来看一个使用了DPM刻印二维码的医疗器械进行追溯管理的医院的案例。这些操作通常在医院不被重视。然而,手术环节恶劣的卫生条件,极有可能造成大混乱,比如中断等手术或继发感染的风险。当然,在最糟糕的情况下,可能造成死亡。
因此,采用了追溯系统,医院不但可以提高工作效率,而且也可避免依赖于个人能力,从而真正地保障了患者的人身安全。
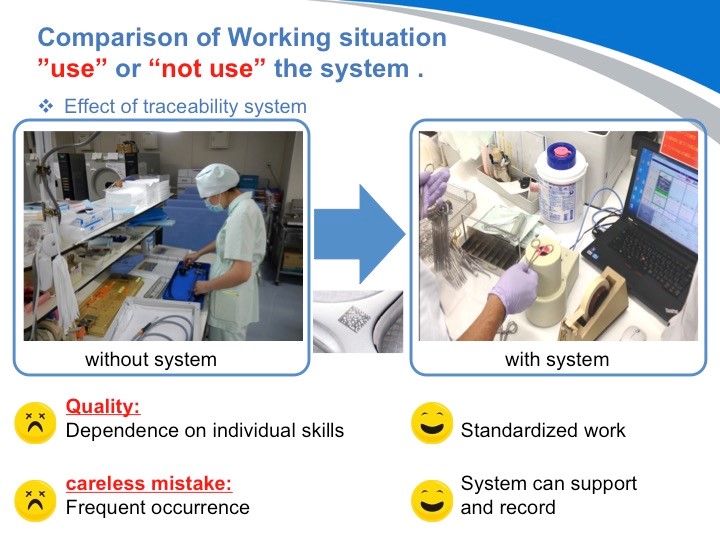
请看这张图:追溯管理系统不仅仅只是将确保患者安全的操作标准化,同时还能产生更大的效应。
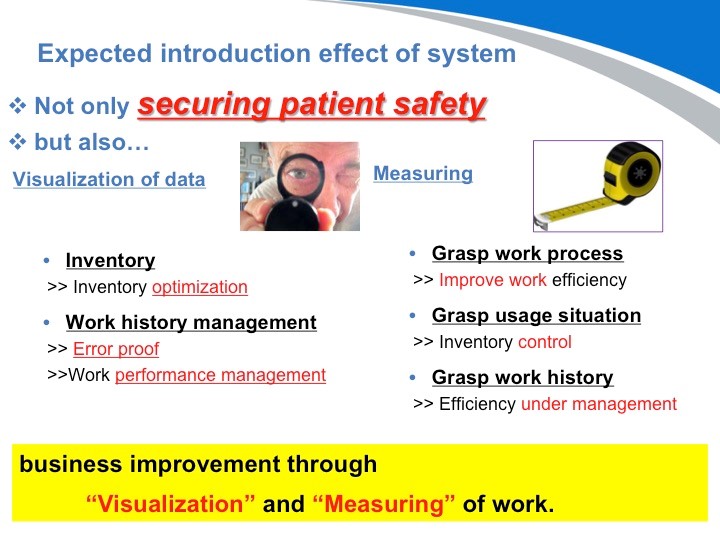
DPM毕竟只是起到识别的作用。但是采用了这样的通过识别而实现的医疗器械的追溯系统,才能真正将数据可视化。不单如此,将至今为止无法看到的数据识别后,更能正确的把握实际运营情况,从而实现业务的改善。

好,我们介绍了什么是DPM,以及介绍了采用DPM对手术器械进行追溯管理可以对医院管理上带来巨大的效应。那我现在要给大家介绍的是,既然我们可以对器械进行管理和追溯,那同样我们也可以从中发现有用的和不需要的物品,从而对医院的仓储管理起到决定性作用。
我认为医院的管理方法取决于医院的规模和经营理念。也就是说,不是所有的医院都有必要进行单件物品的管理和追溯。但是我要说的是,对于单件物品进行管理不但能确保患者的安全,同时它能产生更大更多的利益。下面我给大家列举几个实际在日本使用单件物品追溯管理的医院案例,以及它给医院带来的经济效应。

这里,我列举了3家在日本非常有名的医院。这些医院都按照技术指南的要求实施了DPM刻印进行医疗器械的追溯管理。

首先是这家采用了追溯管理系统的医院。
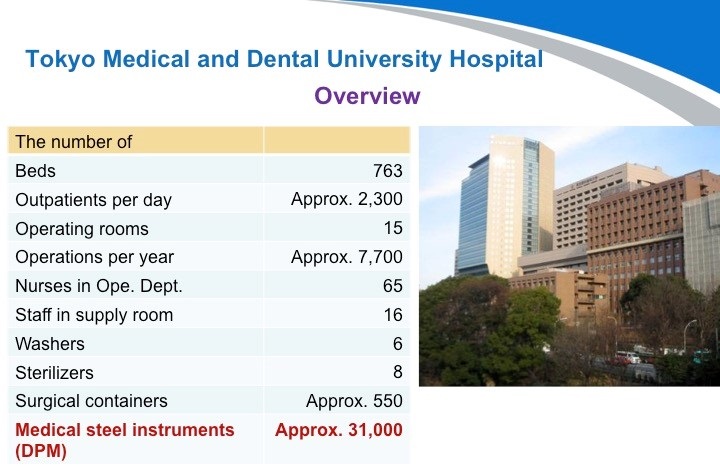
第一家医院:东京医科齿科大学附属医院。这是一家历史非常悠久的医院,位于日本的首都东京。他们有大约550个刻有DPM标识的手术容器(手术包)和约31000把医疗器械。他们积极地采用了可追溯系统,并在所有的容器上采用了RFID无线射频技术进行管理。可以说在医院里的所有医疗器械都受到严格监控。
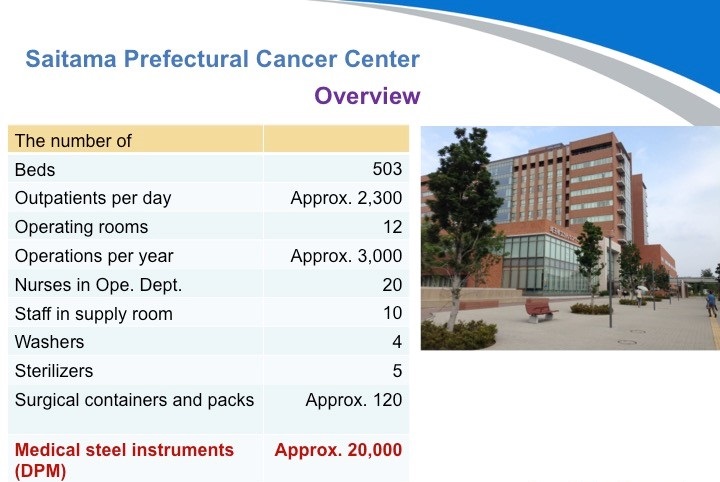
第二家医院:埼玉县癌症中心。位于东京的北部,他们有大约120个刻有DPM标识的手术容器(手术包)和约20000把医疗器械。他们于2013年医院翻新时导入了追溯管理系统。目前正着力于改善业务性能,他们已经开始体会到系统带来的积极效应。
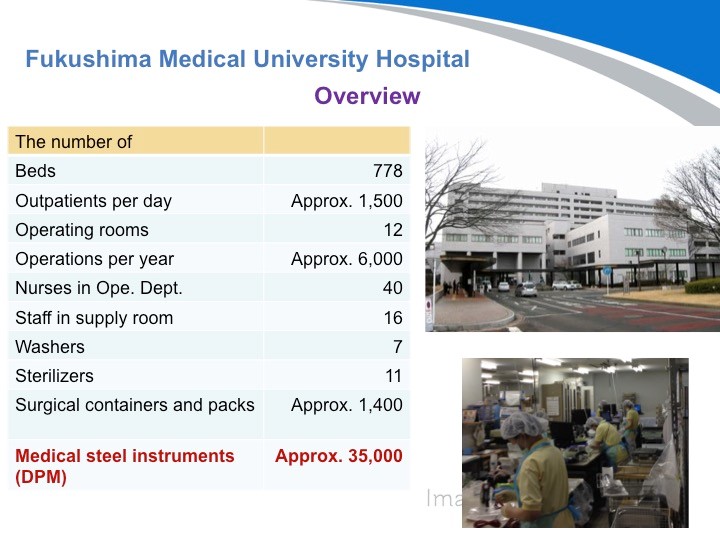
第三家医院:福島県立医科大学附属医院是当地最知名的医院。他们有大约1,400个刻有DPM标识的手术容器(手术包)和35000把医疗器械。还有很多医院都导入了这个系统,目前刚刚开始运营。今后如果有机会的话,我可以列举更多的案例给到大家参考。
下面,我将详细地给大家介绍一下这个NTT东日本关东医院的案例。落合医生,曾经在这家医院就职,由于他对医院的杰出贡献,目前仍被该医院任命为名誉主任。在得到了他的允许下,今天我才能分享这个事例给大家。
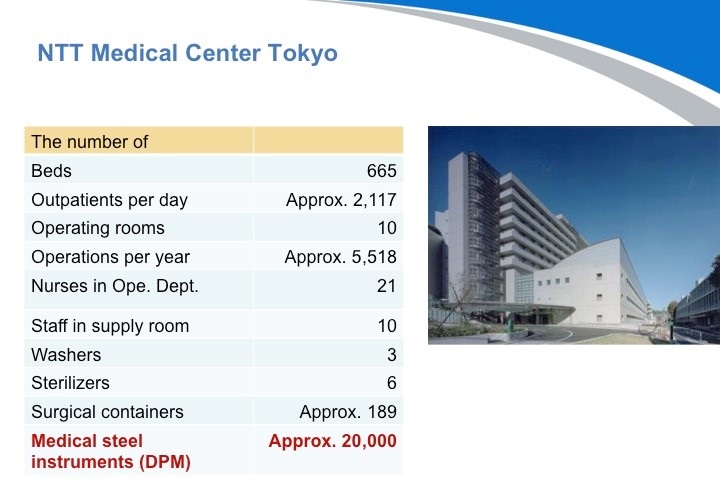
NTT东日本关东医院和我刚才介绍的东京医科齿科大学一样都是坐落在东京。NTT东日本关东医院有约700张床,每天大约有2200名门诊病人,他们有10个手术室,每年的手术量为约6000个。他们有大约189个刻有DPM标识的手术容器和20000把医疗器械。
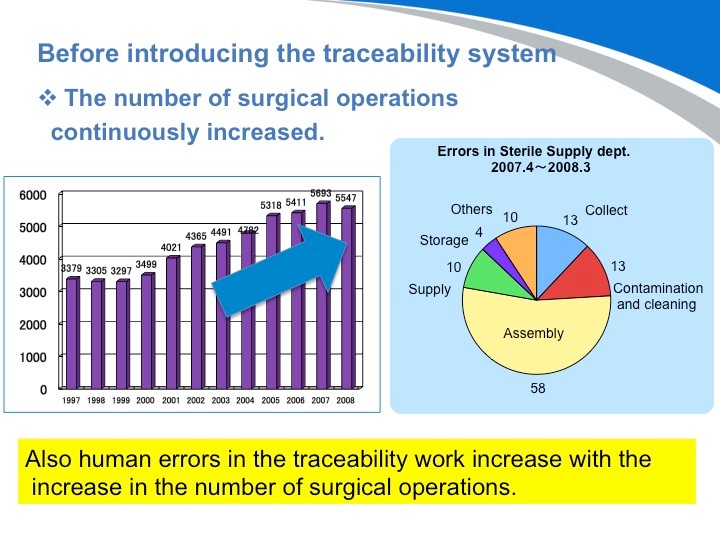
这张图您可以看出,随着工作效率的不断提高,外科手术的数量也在不断增加。但同样消毒供应中心的出错率也在不断地上升。
因此,医院需要尽快分析消毒供应流程,消除人为错误迫在眉睫。那么您认为最有效的措施是什么?首先想到的办法就是,“增加消毒供应中心的人手”。然而,它不是非常有效的方法。由于员工的工作空间受限,工作效率将不可避免地下降,从而质量指数也将进一步恶化。有一件事是肯定的:增加员工的数量,一定会增加医院的运营成本。 他们考虑了很多方法:“什么操作在哪里执行?”医院里到底有多少医疗仪器和器械?什么样的操作容易出错?“”

在这个调查中,为了提高工作效率, NTT东日本关东医院发现他们并没有掌握使用情况。然后,他们做了个重大决定,提出了向病人提供优质的医疗服务的口号。如何做到呢?那就是导入可追溯管理系统。那是我在现场给予他们技术支持的画面仍然记忆犹新。
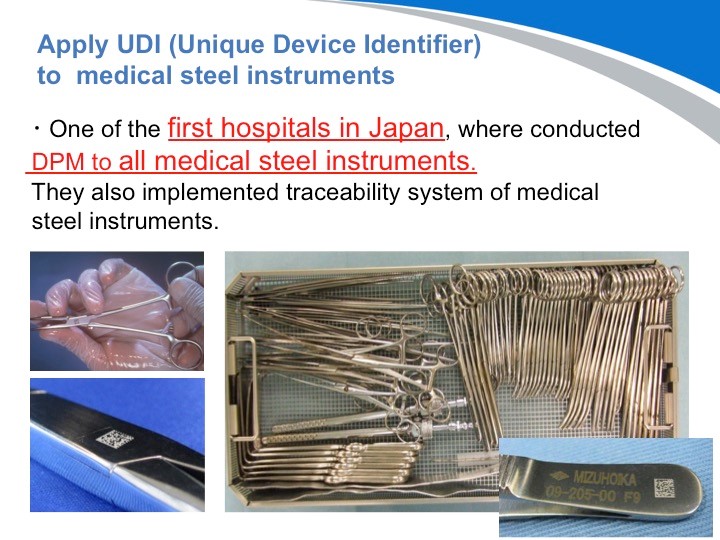
请看这些照片!据我所知, NTT东日本关东医院是日本最先实施DPM二维码刻印的医院。当时我也曾在关东医院协助他们一起刻印手术器材。
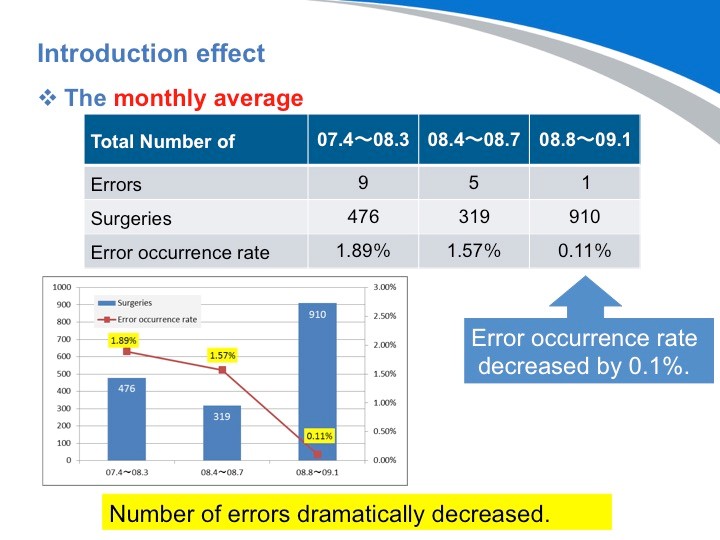
让我们来看看情况是如何转变的。导入系统后,出错率降低到了0.1%。我很坚信,这个数字肯定可以变为零。

导入可追溯系统后,什么变成了可见的呢?
可追溯系统最初被引入这个概念,是为了确保高质量的医疗服务和病人安全。不仅如此,随着系统的导入,其他的一系列问题也变得越来越清晰。下面,我将向大家展示,根据使用情况的分析结果,能带来怎样的效应。
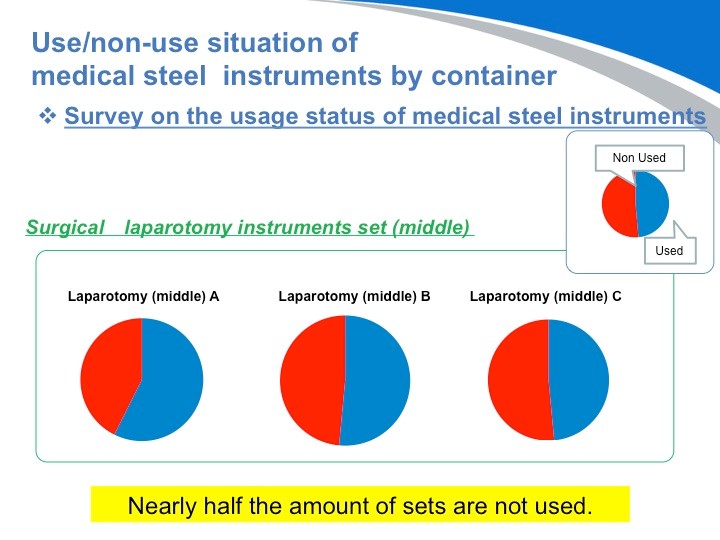
首先我们看到的问题是医疗器械使用上的问题。这个图显示的是使用和未使用情况。蓝色表示医疗器械“使用”, 红色表示医疗器械“未使用”。同样的手术,不同的医生显示的医疗器械有一半是用不到的。
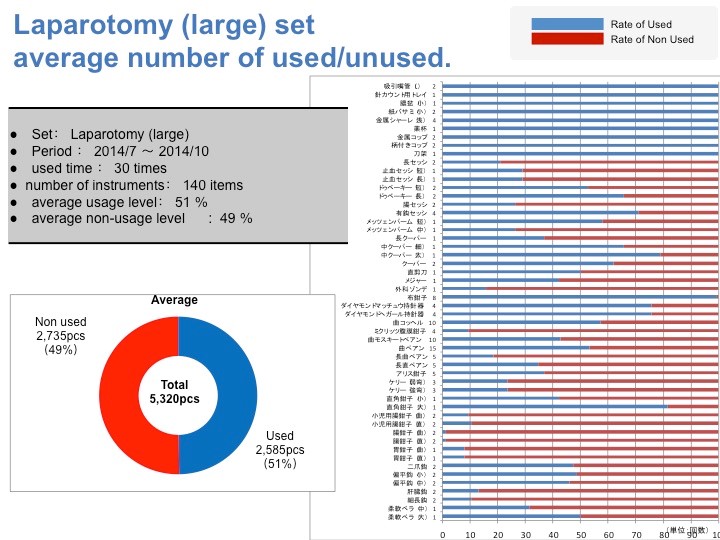
我试图分析了一下刚才介绍的器械使用情况的细分特征和倾向。这个图表非常复杂,所以我们只要来看一下趋势。Y轴显示的是器械的名称。柱状图显示了该器械的使用比例。蓝线表示使用。结果我们可以把他们分成3种类型:1. 必须的2. 有比没有好3. 完全不需要。
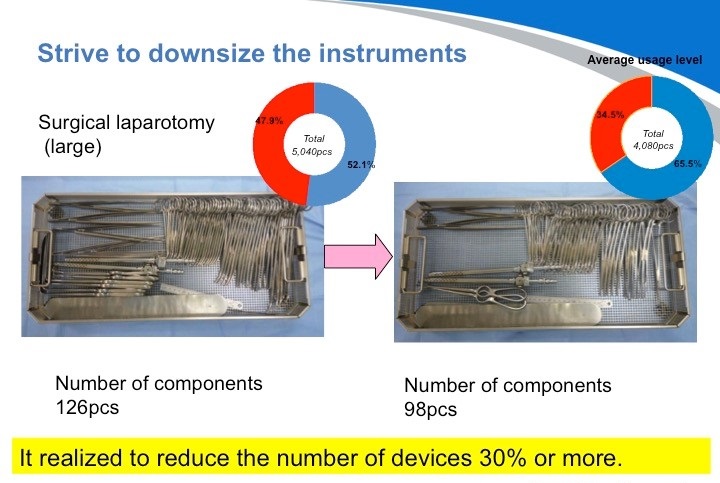
结果就是,我们可以把手术器材重新组合,最终成功了减少了30%的浪费。
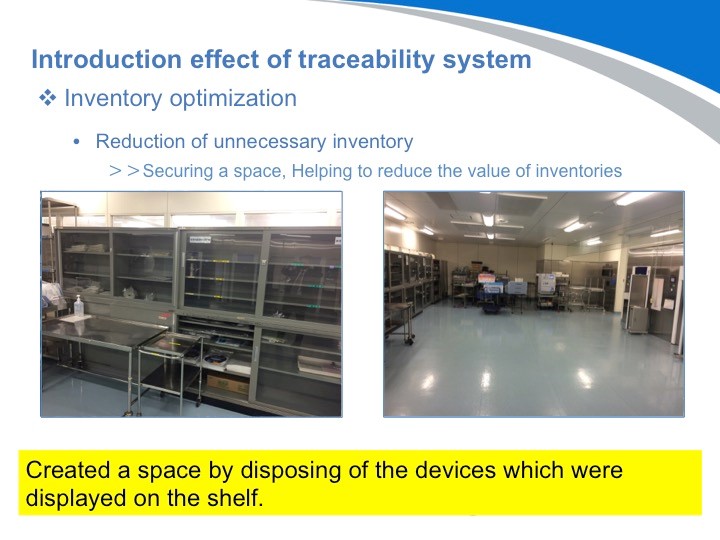
下一个好处是库存的优化。由于采用了可追溯管理系统,刚才整理出来的不必要的器材就可以处理掉,腾出更多的空间,有效利用起来。

库存优化节省了器械消毒和组装上所花费的时间和精力,带来更多的经济效益。从这张图表上可以看出,但他们成功地降低了原本不断增长的劳动力成本。且手术的数量并没有变化。此外,由于减少了存货,医院的运营成本也降低了不少。
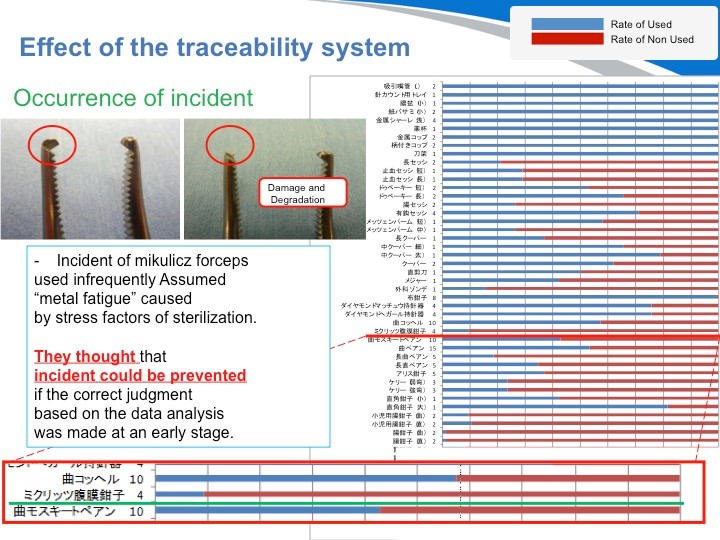
下面,我要介绍另一个单件器械管理的实例。这是在日本发生的一起真实的医疗事故。按以往的调查方式,最终结论只会是器材不良、老化裂化等结果。但由于采用了单件器械管理,我们就能得出其他不同的见解。这个医疗事故是由一把刀头断裂的手术器材而引发的。从数据上显示得知,这把是几乎从来都没用过的手术器材。
也就是说,并非由于手术使用而损耗,而是由于虽然并未使用过却被反复清洗和消毒,导致的金属裂化。最终采取的措施是,将其从手术包明细里去除,改用单品包装,确保需要的时候能立即提取的管理方式。若是当初就有数据可以进行可视化管理和分析,就能将这样的医疗事故防范于未然。

在此,我想再强调一下,可追溯管理系统的导入不单可以提升经济效益,更可以通过数据分析确保患者的人生安全。今天,我谈到了DPM技术,UDI监管要求及其作用。DPM配套的可追溯管理系统才刚刚起步。而DPM二维码刻印的需求已经在世界各地蔓延。我今天只能介绍一部分信息,但相信随着形势的发展,您将逐渐获得更多信息。